ULTOMIRIS® Efficacy was evaluated for aHUS patients in two Phase III, single arm, open label, multicentre studies1,2,5
C5 Inhibition
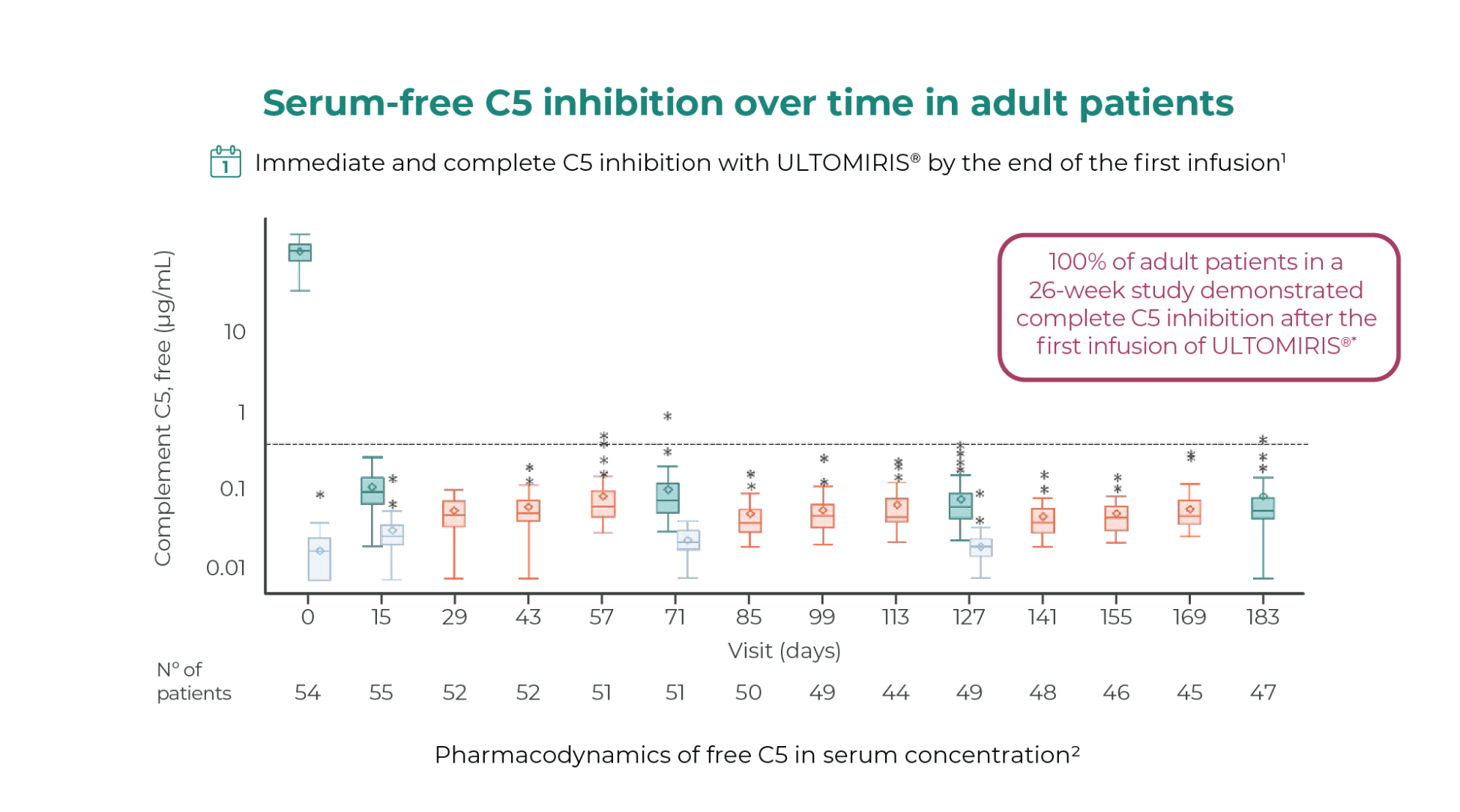
ULTOMIRIS® provided immediate and complete C5 inhibition in adult and pediatric patients* from Day 11,2
p-values were not included in this publication
Adapted from Rondeau E, et al. 2020.
Horizontal line is drawn at free C5 at 0.5 ug/ml to denote the threshold for complete terminal complement inhibition. The horizontal line in the middle of each box indicates the median, a diamond indicates the mean, and the top border and the bottom border of the boxes mark the 75th and 25th percentiles, respectively. The whiskers represent the highest and lowest values within 1.5 the interquartile range from the lower quartile and upper quartile. Outliers are represented by an asterisk beyond the whiskers.
This clinical study was based on the administration of ULTOMIRIS® 10 mg/mL.
Number of patients defined by the available data for each specific assessment at Day 183.8.
*As defined by free C5 in serum concentrations less than 0.5 μg/mL.8.
BL, baseline; EOI, end of infusions; MD, maintenance dose.
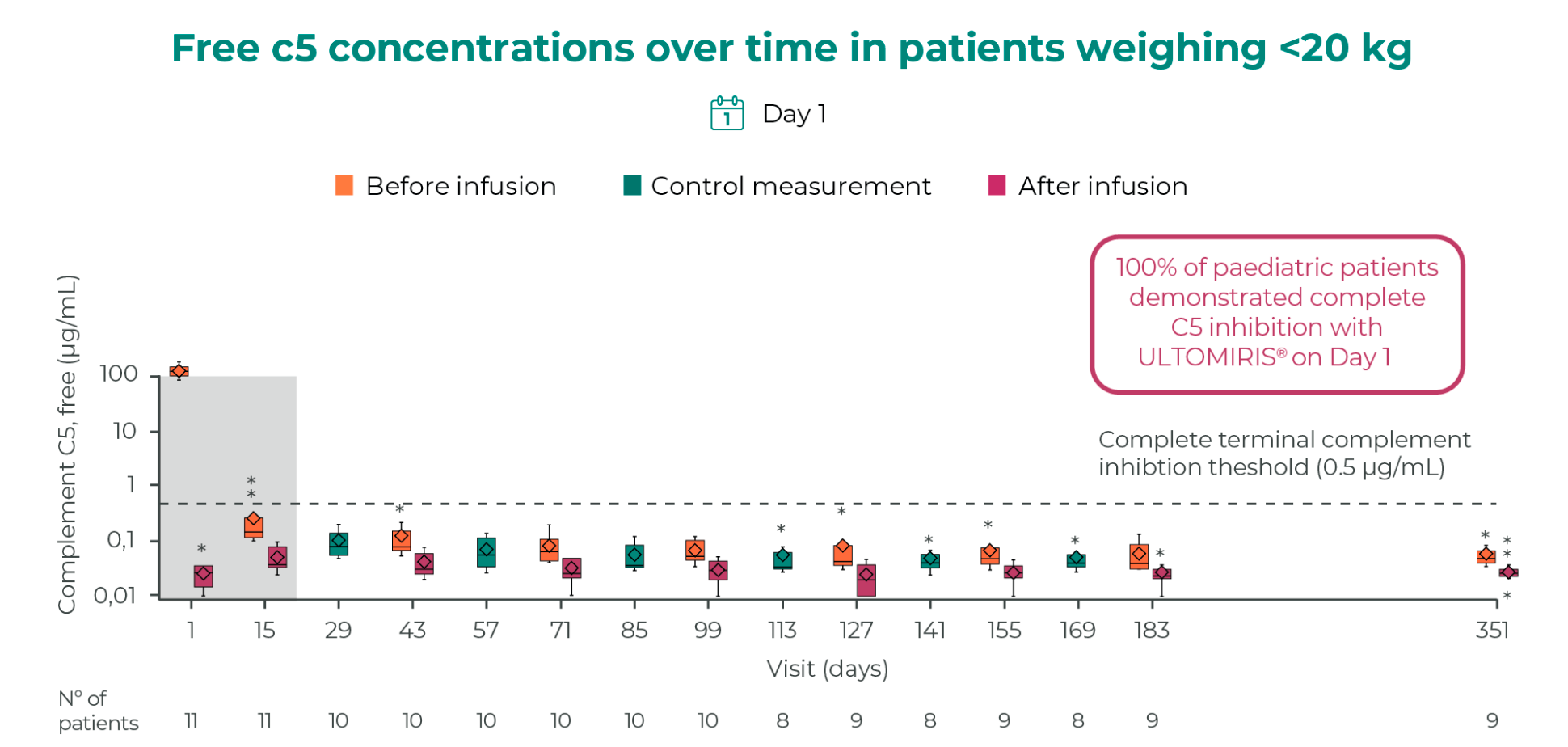
ULTOMIRIS® provides immediate and complete C5 inhibition from day 1 in pediatric patients2
p-values were not included in this publication
Modified from Ariceta G, et al. 2021.3 Data displayed as mean ± SD.
This clinical study was based on the administration of ULTOMIRIS® 10 mg/mL. Free C5 levels shown at the original loading dose of 300 mg for patients <10 kg. Horizontal line is drawn at free C5 at 0.5 mg/ml to denote the threshold for complete terminal complement inhibition. The horizontal line in the middle of each box indicates the median, a diamond indicates the mean, and the top border and the bottom border of the boxes mark the 75th and 25th percentiles, respectively. The whiskers represent the highest and lowest values within 1.5 times the interquartile range from the lower quartile and upper quartile. Outliers are represented by an asterisk.
TMA Response
ULTOMIRIS® provided complete TMA response in a majority of the adult patients during 26-week initial evaluation period1,5
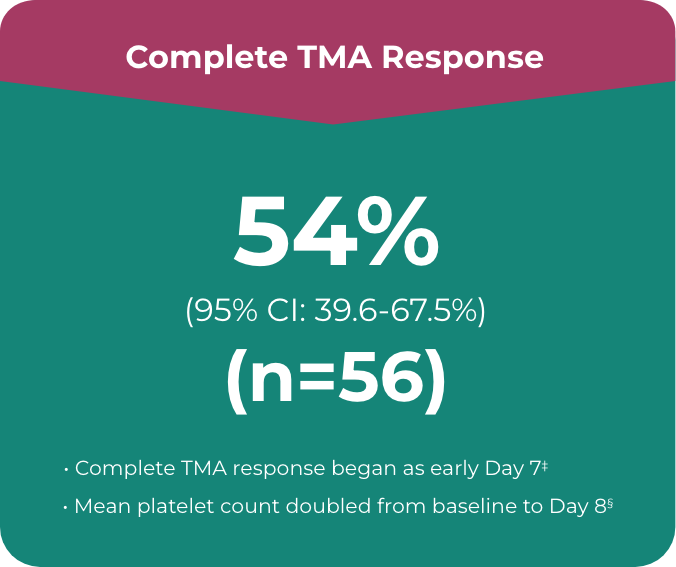
Primary endpoint in the adult study was complete TMA response, which comprised:
- • Platelet count normalisation†
- • Serum LDH normalisation†
- • ≥25% improvement in serum creatinine from baseline
Patients had to meet all criteria for the primary endpoint of a complete TMA response at 2 separate assessments obtained at least 4 weeks (28 days) apart and any measurement in between
ULTOMIRIS®: Complete TMA response in a majority of the adult patients after the 26-week initial evaluation period (n=56)1,5
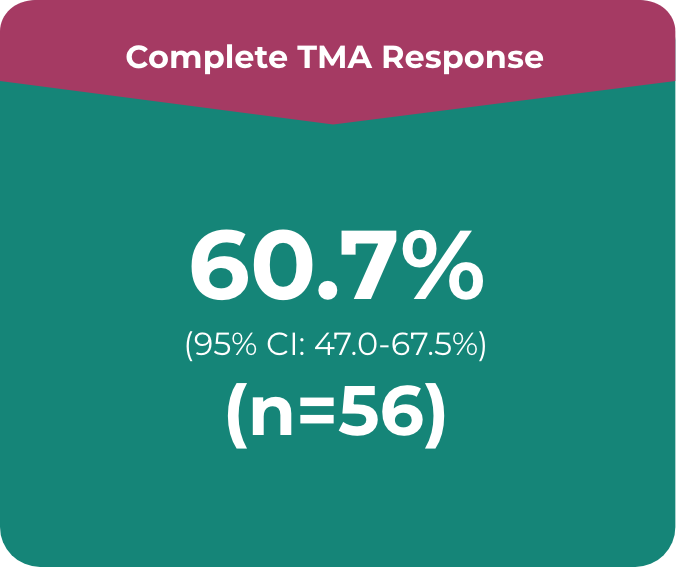
Primary endpoint in the adult study was complete TMA response, which comprised:
- • Platelet count normalisation
- • Serum LDH normalisation
- • ≥25% improvement in serum creatinine from baseline
Patients had to meet all criteria for the primary endpoint of a complete TMA response at 2 separate assessments obtained at least 4 weeks (28 days) apart and any measurement in between
† Normalisation includes ≥150 x 109/L for platelet count and ≤246 U/L for LDH.
‡ Median time to response was 86 days (range, 7 to 169 days) in the 26-week trial.
§ Mean platelet count at Day 8 was 240.34 x 109/L vs 118.52 x 109/L at baseline.
ULTOMIRIS®: Complete TMA response in a majority of the pediatric patients during 26-week initial evaluation period (n=18)2
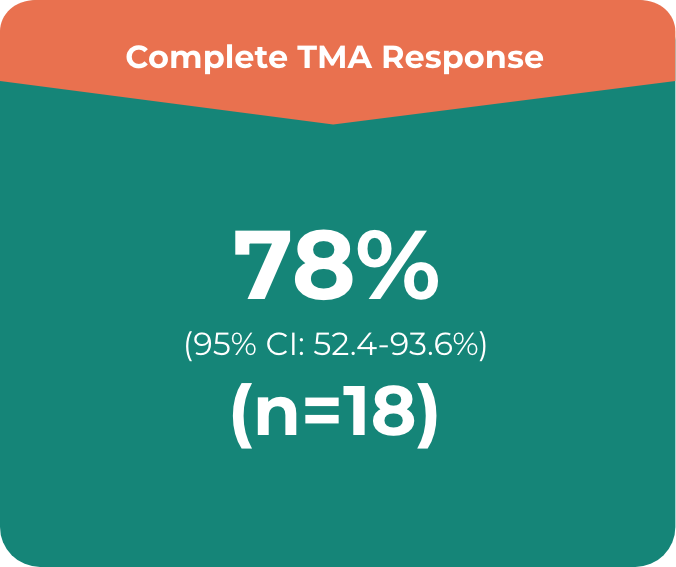
Primary endpoint in the adult study was complete TMA response, which comprised:
- • Platelet count normalisation
- • Serum LDH normalisation
- • ≥25% improvement in serum creatinine from baseline
Patients had to meet all criteria for the primary endpoint of a complete TMA response at 2 separate assessments obtained at least 4 weeks (28 days) apart and any measurement in between
ULTOMIRIS®: Complete TMA response in a majority of the pediatric patients after the 26-week initial evaluation period (n=18)2
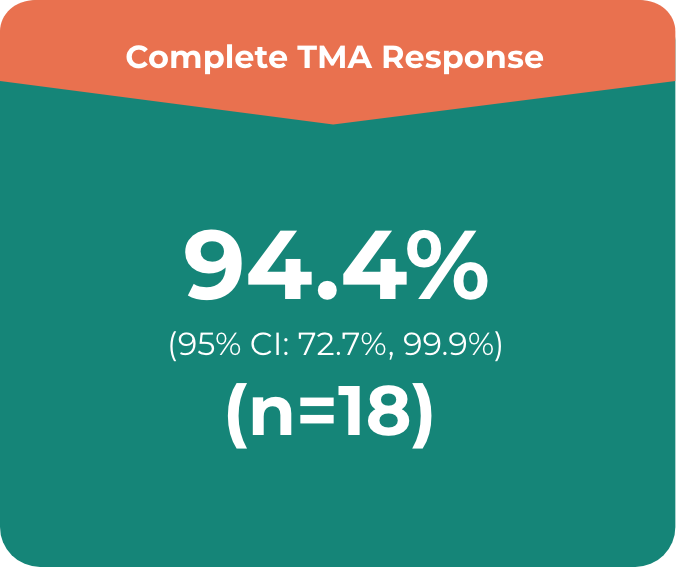
Primary endpoint in the adult study was complete TMA response, which comprised:
- • Platelet count normalisation
- • Serum LDH normalisation
- • ≥25% improvement in serum creatinine from baseline
Patients had to meet all criteria for the primary endpoint of a complete TMA response at 2 separate assessments obtained at least 4 weeks (28 days) apart and any measurement in between
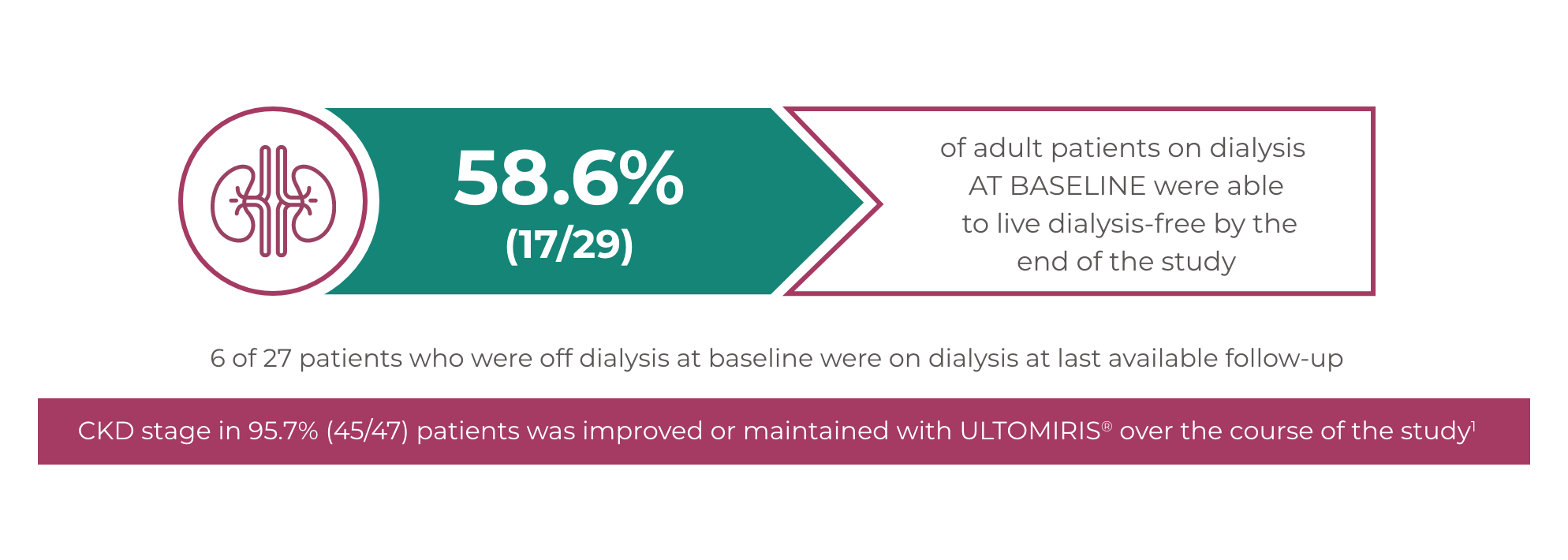
Dialysis-free
ULTOMIRIS®: An opportunity to live dialysis-free for adult patients1,5
ULTOMIRIS®: An opportunity to live dialysis-free for pediatric patients2
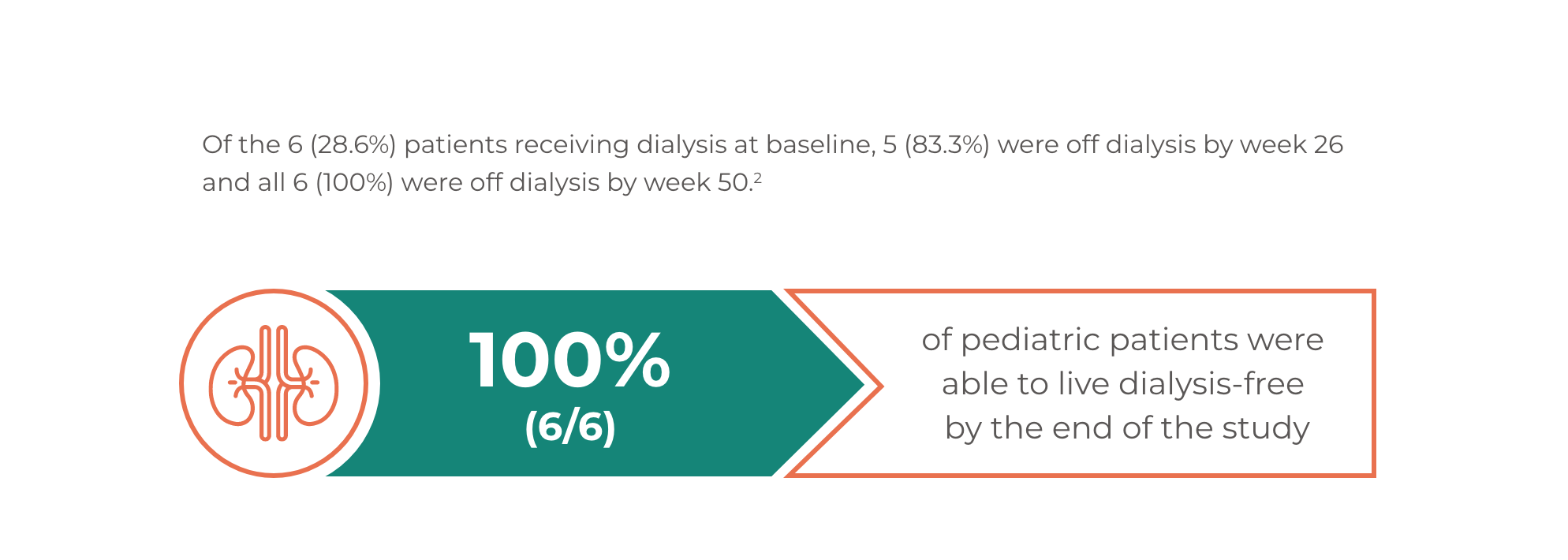
ULTOMIRIS® Efficacy in Pediatric Patients with aHUS previously treated with eculizumab4,5
In eculizumab-experienced patients, switching to ULTOMIRIS® maintained disease control as evidenced by stable hematologic and renal parameters, with no apparent impact on safety5
Adverse events
Summary of adverse events through the current data-cuta (safety set)
Overall (N=10)
Adverse event term
N (%)
Events
Any AE
10 (100)
66
Any serious AE
1 (10.0)
5
TESAEs resulting in drug discontinuation
0
0
TEAEs resulting in trial discontinuation
0
0
TEAEs during trial drug infusion
2 (20.0)
4
TESAEs during trial drug infusion
0
0
Treatment-related AEs
2 (20.0)
4
Meningococcal infections
0
0
Deaths
0
0
Most frequent treatment-emergent adverse events
Overall (N=10)
N (%)
Events
Upper respiratory tract infection
4 (40.0)
19
Upper respiratory tract infection
2 (20.0)
2
Otitis media
2 (20.0)
2
Pharyngitis
2 (20.0)
2
Viral upper respiratory tract infection
2 (20.0)
2
Oropharyngeal pain
3 (30.0)
3
Adapted from Tanaka K, et al. 2021.
Events occurring in >15% of patients listed. Adverse event terms are as reported by the treating investigator. Patients evaluated for safety included all patients that received ≥ 1 dose of the study drug.


